An enantiospecific route towards taiwaniaquinoids. First synthesis of (−)-taiwaniaquinone H and (−)-dichroanone†
Abstract
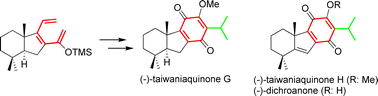
A new methodology for the enantiospecific synthesis of taiwaniaquinoids, based on a thermal 6π electrocyclization, is reported. Under this procedure, 4a-methylhexahydrofluorene terpenoids bearing an A/B trans-configuration has been prepared for the first time. This methodology also makes it feasible to synthesize taiwaniaquinoids with an A/B cis-configuration and 4a-methyltetrahydrofluorene terpenoids. Accordingly, the first synthesis of (−)-taiwaniaquinone G, (−)-taiwaniaquinone H and (−)-dichroanone has been achieved.

 Please wait while we load your content...
Please wait while we load your content...