Abstract
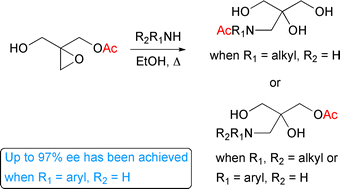
The synthesis of a number of β-amino tertiary alcohols has been achieved via ring-opening of an unsymmetrical epoxide with primary and secondary amines. The results revealed that primary amines give symmetrical triol products following an undesired acyl migration reaction, whereas secondary amines give the desired chiral (racemic) products. Furthermore, we demonstrate that asymmetric products can be formed using enantiomerically pure epoxide and aromatic amines without any loss of enantiomeric excess.

 Please wait while we load your content...
Please wait while we load your content...