Expedient synthesis of 3-hydroxyisoquinolines and 2-hydroxy-1,4-naphthoquinones via one-pot aryne acyl-alkylation/condensation†
Abstract
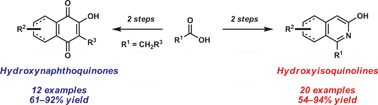
A convenient method is disclosed for the synthesis of both 3-hydroxyisoquinolines and 2-hydroxy-1,4-naphthoquinones from β-ketoesters using a one-pot

 Please wait while we load your content...
Please wait while we load your content...