An investigation into the electrophilic cyclisation of N-acyl-pyrrolidinium ions: a facile synthesis of pyrrolo-tetrahydroisoquinolones and pyrrolo-benzazepinones†
Abstract
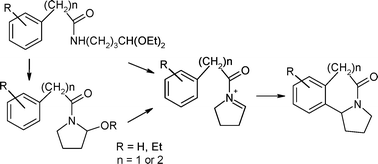
The triflic acid-mediated cyclisation of N-arylmethyl- and N-arylethyl-acylpyrrolidinium ions gave moderate to good yields of pyrrolo-tetrahydroisoquinolones and pyrrolo-benzazepinones respectively. Electron-donating R substituents enhanced the rate of reaction and gave higher yields than electron-withdrawing substituents. Substituents on the

 Please wait while we load your content...
Please wait while we load your content...