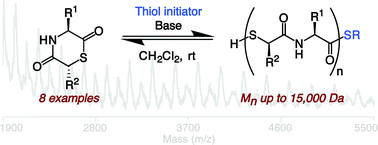
We describe the preparation and characterization of polythioesters composed of alternating α-amino acid and α-thioglycolic acid residues that undergo dynamic constitutional exchange under mild conditions. The polymers are assembled via reversible ring-opening polymerizations of 1,4-thiazine-2,5-diones and related monomers in solution-phase conditions that do not require the use of transition metal catalysts. Because 1,4-thiazine-2,5-diones can be derived in part from α-amino acids, a variety of side chain functionalized monomers in optically pure forms could readily be accessed. In addition, the resulting polythioesters have the potential for intra- and inter-chain hydrogen bonding, which is known to impart materials properties to other previously studied polyamides. The studies reported here could be useful in advancing a new class of biodegradable polymers and furthermore suggest that dynamic constitutional exchange could be exploited to modify many known synthetic and natural polythioesters.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...