A selective de-O-methylation of guaiacyl lignans to corresponding catechol derivatives by 2-iodoxybenzoic acid (IBX). The role of the catechol moiety on the toxicity of lignans
Abstract
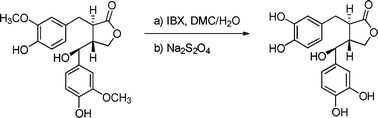
We report here the first selective de-O-methylation of a large panel of guaiacyl

 Please wait while we load your content...
Please wait while we load your content...