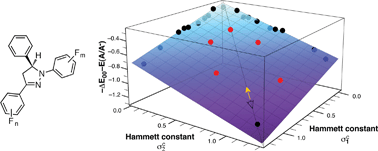
The photophysical properties of 1,3,5-triarylpyrazolines are strongly influenced by the nature and position of substituents attached to the aryl-rings, rendering this fluorophore platform well suited for the design of fluorescent probes utilizing a photoinduced electron transfer (PET) switching mechanism. To explore the tunability of two key parameters that govern the PET thermodynamics, the excited state energy ΔE00 and the acceptor potential E(A/A−), a library of polyfluoro-substituted 1,3-diaryl-5-phenyl-pyrazolines was synthesized and characterized. The observed trends for the PET parameters were effectively captured through multiple Hammett linear free energy relationships (LFER) using a set of independent substituent constants for each of the two aryl rings. Given the lack of experimental Hammett constants for polyfluoro-substituted aromatics, theoretically derived constants based on the electrostatic potential at the nucleus (EPN) of carbon atoms were employed as quantum chemical descriptors. The performance of the LFER was evaluated with a set of compounds that were not included in the training set, yielding a mean unsigned error of 0.05 eV for the prediction of the combined PET parameters. The outlined LFER approach should be well suited for designing and optimizing the performance of cation-responsive 1,3,5-triarylpyrazolines.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...