Synthesis and analytical resolution of chiral pyrazoles derived from (5R)-dihydrocarvone†
Abstract
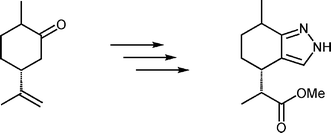
In the course of the development of chiral hydrotris(pyrazolyl)borate
- This article is part of the themed collection: In honour of Jean-Pierre Sauvage

 Please wait while we load your content...
Please wait while we load your content...