Synthesis and properties of oxygen non-stoichiometric BiMnO3
Abstract
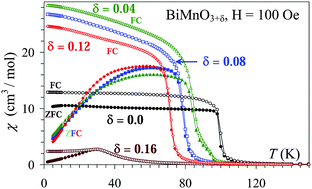
We have investigated oxygen non-stoichiometric BiMnO3±δ samples. It was found that the oxygen deficient samples could not be prepared by the direct high-pressure high-temperature method and other methods should be developed for the preparation of BiMnO3−δ. It was confirmed that stoichiometric BiMnO3 adopts the monoclinic phase I structure (space groupC2/c) in agreement with previous reports, and BiMnO3 is a ferromagnet with TC = 100 K. Single-phase BiMnO3.04, BiMnO3.08, and BiMnO3.12 samples were prepared with the monoclinic phase II structure and ferromagnetic transitions at TC = 85, 80, and 72 K, respectively. However, in the case of BiMnO3.08 and BiMnO3.12, very weak reflections breaking the symmetry of the C-centered monoclinic cell were observed. BiMnO3.16 crystallizes in an orthorhombic system with lattice parameters (a = 5.5143(1) Å, b = 7.8106(2) Å, and c = 5.5614(1) Å in space groupPnma) close to those of LaMnO3.11. BiMnO3.16 shows a spin-glass transition at 30 K.

 Please wait while we load your content...
Please wait while we load your content...