Redox properties of gold-substituted zirconia surfaces
Abstract
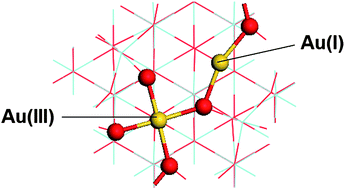
We report the use of quantum mechanical calculations based on the density functional theory (DFT) to investigate the redox properties of zirconia surfaces with cationic gold centres. Two different charge compensation mechanisms for the Au/Zr substitution at the cubic zirconia (111) surface are investigated: formation of oxygen vacancies and surface protonation. Regardless of the mechanism of charge compensation, gold dopants are more likely to accumulate at the surface than to migrate to the zirconia bulk. We investigate the formation of oxygen vacancies in the pure and gold-substituted surfaces, exploring a range of vacancy configurations, and we show that the presence of gold in lattice positions at the zirconia surface induces a dramatic change in the redox properties of the surface, which becomes easily reducible thanks to Au(III) → Au(I) transitions.

 Please wait while we load your content...
Please wait while we load your content...