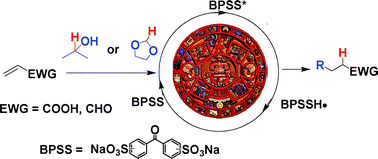
The usefulness of solar light for carrying out photocatalytic reactions involving the formation of a carbon–carbon bond has been explored. Thus, some radical alkylations of α,β-unsaturated acids or aldehydes have been carried out in a mixed aqueous solution. Under these conditions, alkyl radicals are generated from i-PrOH and 1,3-dioxolane by photocatalyzed hydrogen abstraction. A water soluble photocatalyst (disodium benzophenondisulfonate, BPSS) was used, which greatly simplifies work up. With reasonably efficient radical traps (e.g. maleic acid), the syntheses could be carried out up to completion on a 10 gram scale within 10–15 hours exposure to sunlight in a solar concentrator (SOLFIN apparatus) in November in Almeria (Spain). The alkylation of some α,β-unsaturated aldehydes have been likewise performed under the same conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...