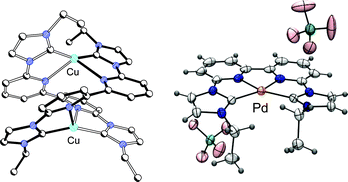
Controlling the coordination modes of a new and highly flexible ligand bearing two N-heterocyclic carbene moieties at a bipyridine backbone†
Abstract
A new and highly flexible biscarbene ligand with two
- This article is part of the themed collection: N-heterocyclic carbenes

 Please wait while we load your content...
Please wait while we load your content...