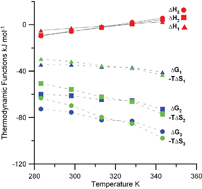
The protonation reactions of oxalate (ox) and the complex formation of uranium(VI) with oxalate in 1.05 mol kg−1 NaClO4 were studied at variable temperatures (10–70 °C). Three U(VI)/ox complexes (UO2oxj(2−2j)+ with j = 1, 2, 3) were identified in this temperature range. The formation constants and the molar enthalpies of complexation were determined by spectrophotometry and calorimetry. The complexation of uranium(VI) with oxalate ion is exothermic at lower temperatures (10–40 °C) and becomes endothermic at higher temperatures (55–70 °C). In spite of this, the free energy of complexation becomes more negative at higher temperatures due to increasingly more positive entropy of complexation that exceeds the increase of the enthalpy of complexation. The thermodynamic parameters at different temperatures, in conjunction with the literature data for other dicarboxylic acids, provide insight into the relative strength of U(VI) complexes with a series of dicarboxylic acids (oxalic, malonic and oxydiacetic) and rationalization for the highest stability of U(VI)/oxalate complexes in the series. The data reported in this study are of importance in predicting the migration of uranium(VI) in geological environments in the case of failure of the engineering barriers, which protect waste repositories.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...