The protonolysis of anionic lanthanide amide complexes (THF)LiLn(NPri2)4 with two equiv. of tridentate Schiff base HL (L = 3,5-But2-2-O-C6H2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-C5H4N) afforded the unanticipated products LiL′2Ln(THF) (L′ = 3,5-But2-2-O-C6H2CH(NPri2)-N-C5H4N; Ln = Y 1, Sm 2 and Yb 3) formed by intramolecular nucleophilic attack of the amide groups at the imine carbon atoms of the original ligand, L. The protonolysis is greatly affected by the amide group. When amine elimination was carried out with a mixture of Ln[N(TMS)2]3(μ-Cl)Li(THF)3 (TMS = SiMe3) and LiN(TMS)2, the expected lanthanide amide complexes with two Schiff base ligands, L2Ln[N(TMS)2] (Ln = Sm 4 and Nd 5), were isolated and no migration of the N(TMS)2 group was observed because of steric hindrance. Complexes 1–5 were well characterized including X-ray structural analyses.
N-C5H4N) afforded the unanticipated products LiL′2Ln(THF) (L′ = 3,5-But2-2-O-C6H2CH(NPri2)-N-C5H4N; Ln = Y 1, Sm 2 and Yb 3) formed by intramolecular nucleophilic attack of the amide groups at the imine carbon atoms of the original ligand, L. The protonolysis is greatly affected by the amide group. When amine elimination was carried out with a mixture of Ln[N(TMS)2]3(μ-Cl)Li(THF)3 (TMS = SiMe3) and LiN(TMS)2, the expected lanthanide amide complexes with two Schiff base ligands, L2Ln[N(TMS)2] (Ln = Sm 4 and Nd 5), were isolated and no migration of the N(TMS)2 group was observed because of steric hindrance. Complexes 1–5 were well characterized including X-ray structural analyses.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-C5H4N) afforded the unanticipated products LiL′2Ln(THF) (L′ = 3,5-But2-2-O-C6H2CH(NPri2)-N-C5H4N; Ln = Y 1, Sm 2 and Yb 3) formed by intramolecular nucleophilic attack of the amide groups at the
N-C5H4N) afforded the unanticipated products LiL′2Ln(THF) (L′ = 3,5-But2-2-O-C6H2CH(NPri2)-N-C5H4N; Ln = Y 1, Sm 2 and Yb 3) formed by intramolecular nucleophilic attack of the amide groups at the 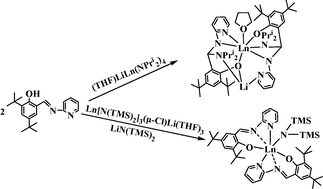

 Please wait while we load your content...
Please wait while we load your content...