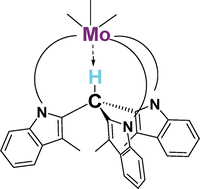
The reaction of HTIMP3 (HTIMP3 = tris[1-diphenylphosphino)-3-methyl-1H-indol-2-yl]methane) with AgBF4 and Mo(CO)3(NCCH3)3 leads to Ag(HTIMP3)BF4 and Mo(CO)3(HTIMP3), respectively. The metal centre is coordinated to the three phosphorus atoms of the HTIMP3 ligand, which adopts a facial coordination mode, placing a H-Csp3hydrogen atom at the apical position close to the metal centre. The solid-state structure of Mo(CO)3(HTIMP3) has been determined by X-ray crystallography, and the data have been used as input parameters for obtaining the optimised geometry of the complex using the B3PW91 functional. The silver structure has been modelled from the X-ray parameters of the molybdenum structure. In addition, theoretical calculations on the H–Csp3 downfield shift upon metal coordination has also been performed. They reproduce the experimental H–Csp3 chemical shifts well and supports that proton deshielding is mainly due to the presence of the metal, since the hydrogen is already located in the cone created by the aromatic-phosphino arms in the free ligand.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...