Experimental charge density in an oxidized trinuclear iron complex using 15 K synchrotron and 100 K conventional single-crystal X-ray diffraction†
Abstract
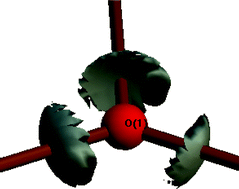
The experimental electron density distribution in a crystal consisting of the simplest conceivable trinuclear carboxylate-bridged iron-μ3-oxo dianion with two α-picolinium

 Please wait while we load your content...
Please wait while we load your content...