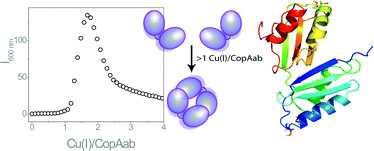
CopA from Bacillus subtilis is a Cu(I)-transporting P-type ATPase involved in resistance to high levels of environmental copper. At its N-terminus are two soluble domains, a and b, that, when generated in isolation from the membrane part, have previously been shown to exhibit unusual Cu(I)-binding behaviour: at >1 Cu(I) per CopAab the protein dimerises, resulting in the formation of a species with luminescence properties characteristic of a solvent-shielded Cu(I) cluster. Further insight into the Cu(I)-binding properties of CopAab are now reported. We demonstrate that the initial binding of Cu(I) occurs with very high affinity (K = ∼4 × 1017 M−1) and that CopAab can accommodate up to 4 Cu(I) per protein and remains dimeric at higher Cu(I)-loadings. Fitting of UV-visible, near UV CD, fluorescence and luminescence spectroscopic titration data supports a model in which Cu(I) binds sequentially to CopAab, and also provides estimates of the association constants for Cu(I)-binding and dimerisation steps. Finally, low molecular weight thiols are shown not to affect the initial binding of Cu(I), but significantly influence binding at levels >1 Cu(I) per CopAab such that dimerisation is inhibited, though not abolished.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...