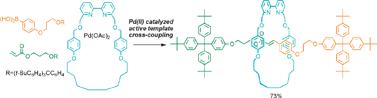
Active metal template synthesis is a powerful new strategy for the construction of rotaxanes, catenanes and other mechanically interlocked molecular structures. The key feature is that the metal plays a dual role during the assembly of the interlocked architecture, acting as both a template for entwining or threading the components and as a catalyst for capturing the interlocked final product by covalent bond formation. Unlike traditional “passive” metal template methods to rotaxanes and catenanes, permanent recognition motifs are not required on each of the components to be interlocked (i.e., the assembly can be traceless) and the template can often be used in sub-stoichiometric quantities. Since its inception in 2006, a rapidly growing number of different metal-catalysed reactions have proven suitable for the active metal template synthesis of both rotaxanes and catenanes, including the copper(I)-catalysed terminal alkyne–azide cycloaddition (the CuAAC “click” reaction), palladium- and copper-catalysed alkyne homocouplings and heterocouplings, and palladium-catalysed oxidative Heck couplings and Michael additions. In addition to simple rotaxanes and catenanes, the synthetic strategy has been used to construct switchable molecular shuttles with weak intercomponent interactions (a requirement for fast shuttling) and to provide insight into the mechanisms of transition metal-catalysed reactions. In this tutorial review we highlight the utility and potential of the early examples of the active metal template strategy in mechanically interlocked molecule synthesis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...