Sigmatropic rearrangements of ‘onium’ ylids
Abstract
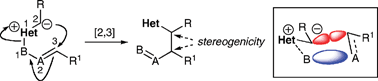
Rearrangement reactions occupy a special place within the canon of organic synthesis, by virtue of the inherently high efficiency of chemical processes which form and break bonds by redistribution of electrons around a retained atomic framework. Within the broader class, sigmatropic rearrangements are chemical processes defined by mechanisms involving unimolecular migration of σ-bonds with concomitant redistribution of one or more π-bonds. Sigmatropic processes may involve uncharged or charged species, with the charges located on carbon or heteroatoms; the latter reaction type is the subject of this tutorial review.

 Please wait while we load your content...
Please wait while we load your content...