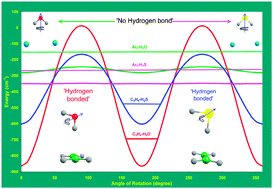
In this manuscript, we propose a criterion for a weakly bound complex formed in a supersonic beam to be characterized as a ‘hydrogen bonded complex’. For a ‘hydrogen bonded complex’, the zero point energy along any large amplitude vibrational coordinate that destroys the orientational preference for the hydrogen bond should be significantly below the barrier along that coordinate so that there is at least one bound level. These are vibrational modes that do not lead to the breakdown of the complex as a whole. If the zero point level is higher than the barrier, the ‘hydrogen bond’ would not be able to stabilize the orientation which favors it and it is no longer sensible to characterize a complex as hydrogen bonded. Four complexes, Ar2–H2O, Ar2–H2S, C2H4–H2O and C2H4–H2S, were chosen for investigations. Zero point energies and barriers for large amplitude motions were calculated at a reasonable level of calculation, MP2(full)/aug-cc-pVTZ, for all these complexes. Atoms in molecules (AIM) theoretical analyses of these complexes were carried out as well. All these complexes would be considered hydrogen bonded according to the AIM theoretical criteria suggested by Koch and Popelier for C–H⋯O hydrogen bonds (U. Koch and P. L. A. Popelier, J. Phys. Chem., 1995, 99, 9747), which has been widely and, at times, incorrectly used for all types of contacts involving H. It is shown that, according to the criterion proposed here, the Ar2–H2O/H2S complexes are not hydrogen bonded even at zero kelvin and C2H4–H2O/H2S complexes are. This analysis can naturally be extended to all temperatures. It can explain the recent experimental observations on crystal structures of H2S at various conditions and the crossed beam scattering studies on rare gases with H2O and H2S.

 Please wait while we load your content...
Please wait while we load your content...