We have used solid-state 17O NMR experiments to measure the 17O quadrupole coupling (QC) and chemical shift (CS) tensors for two α-keto acids: sodium [2-17O]pyruvate and lithium [2,2′-17O2]pyruvate. In the solid state, sodium [2-17O]pyruvate is in the keto form (–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 17O)–) whereas lithium [2,2′-17O2]pyruvate takes the gem-diol form (–C(17OH)2–). This study represents the first time that a full set of 17O NMR tensors are experimentally determined for α-keto acids in these two different tautomeric forms. We have found that the two forms exhibit drastically different 17O QC and CS tensors: for the keto form, δiso = 543 ± 1 ppm, CQ = 10.8 ± 0.2 MHz, ηQ = 0.48 ± 0.05, δ11 = 1020 ± 10, δ22 = 640 ± 10, δ33 = −40 ± 10 ppm, α = 80 ± 5°, β = 90 ± 2°, and γ = 83 ± 2°; for the gem-diol form, δiso = 62 ± 1 ppm, CQ = 8.5 ± 0.5 MHz, ηQ = 1.0 ± 0.05, δ11 = 140 ± 5, δ22 = 45 ± 5, δ33 = 0 ± 5 ppm, α = 55 ± 5°, β = 90 ± 5°, and γ = 80 ± 2°. The 17O chemical shift tensor observed for the gem-diol functional group also represents the first such measurement for any -ol functional group (e.g., alcohols, phenols, carbohydrates, etc.) Using these accurate experimental 17O NMR tensors, we were able to evaluate the accuracy of quantum chemical calculations. Our results showed that quantum chemical calculations using the crystal lattice approach are in much better agreement with the experimental solid-state 17O NMR data than those calculated using the molecular cluster approach. Quantum chemical calculations have also provided information about the sign of the 17O quadrupolar coupling constants and about the 17O NMR tensor orientations in the molecular frame of reference. Our findings suggest that solid-state 17O NMR may be useful in probing the tautomeric form of the α-keto functional group commonly found in intermediates of enzymatic reactions.
17O)–) whereas lithium [2,2′-17O2]pyruvate takes the gem-diol form (–C(17OH)2–). This study represents the first time that a full set of 17O NMR tensors are experimentally determined for α-keto acids in these two different tautomeric forms. We have found that the two forms exhibit drastically different 17O QC and CS tensors: for the keto form, δiso = 543 ± 1 ppm, CQ = 10.8 ± 0.2 MHz, ηQ = 0.48 ± 0.05, δ11 = 1020 ± 10, δ22 = 640 ± 10, δ33 = −40 ± 10 ppm, α = 80 ± 5°, β = 90 ± 2°, and γ = 83 ± 2°; for the gem-diol form, δiso = 62 ± 1 ppm, CQ = 8.5 ± 0.5 MHz, ηQ = 1.0 ± 0.05, δ11 = 140 ± 5, δ22 = 45 ± 5, δ33 = 0 ± 5 ppm, α = 55 ± 5°, β = 90 ± 5°, and γ = 80 ± 2°. The 17O chemical shift tensor observed for the gem-diol functional group also represents the first such measurement for any -ol functional group (e.g., alcohols, phenols, carbohydrates, etc.) Using these accurate experimental 17O NMR tensors, we were able to evaluate the accuracy of quantum chemical calculations. Our results showed that quantum chemical calculations using the crystal lattice approach are in much better agreement with the experimental solid-state 17O NMR data than those calculated using the molecular cluster approach. Quantum chemical calculations have also provided information about the sign of the 17O quadrupolar coupling constants and about the 17O NMR tensor orientations in the molecular frame of reference. Our findings suggest that solid-state 17O NMR may be useful in probing the tautomeric form of the α-keto functional group commonly found in intermediates of enzymatic reactions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 17O)–) whereas lithium [2,2′-17O2]pyruvate takes the gem-
17O)–) whereas lithium [2,2′-17O2]pyruvate takes the gem-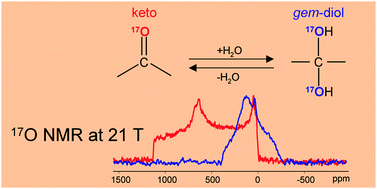

 Please wait while we load your content...
Please wait while we load your content...