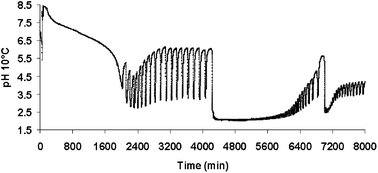
This paper reports the influence of reaction temperature on the occurrence and characteristics of pH oscillations that are observed during the palladium-catalysed phenylacetylene oxidative carbonylation reaction in a catalytic system (PdI2, KI, air, NaOAc) in methanol. Isothermal experiments were performed over the temperature range 10–50 °C. The experiments demonstrate that oscillations occur in the range 10–40 °C and that a decrease in reaction temperature results in an increase in the period and amplitude of the pH oscillations. Furthermore, it is observed that during oscillations at any specific temperature, the time taken for pH to increase from a minimum to a maximum value varies with respect to reaction time. However, the time required for the pH to fall from maximum to new minimum is approximately constant with respect to the reaction time and is a function of the reaction temperature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...