An integrated experimental and theoretical investigation on Cu(hfa)2·TMEDA: structure, bonding and reactivity†
Abstract
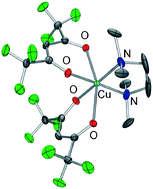
The physico-chemical properties of the β-diketonate diamine Cu(II) compound with hfa (1,1,1,5,5,5-hexafluoro-2-4-pentanedionate) and TMEDA (N,N,N′,N′ tetramethylethylenediamine), Cu(hfa)2·TMEDA, have been thoroughly investigated via an integrated multi-technique experimental–computational approach. In the newly found orthorhombic polymorph, as revealed by low temperature single-crystal X-ray studies, the complex is present as a monomer with a distorted octahedral geometry at the Cu(II) centre. The compound sublimates, without premature side decompositions, at 343 K and 10−3 Torr. The structural, vibrational, electronic and thermal behavior of the neutral Cu(hfa)2·TMEDA complex has been investigated along with its fragmentation pathways, initiated by the release of an anionic hfa

 Please wait while we load your content...
Please wait while we load your content...