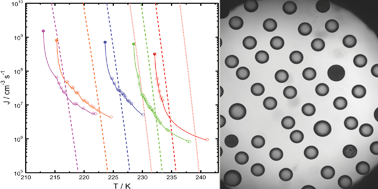
Homogeneous ice nucleation from micrometre-sized aqueous (NH4)2SO4 and aqueous levoglucosan particles is studied employing the optical microscope technique. A new experimental method is introduced that allows us to control the initial water activity of the aqueous droplets. Homogeneous ice freezing temperatures and ice melting temperatures of these aqueous solution droplets, 10 to 80 μm in diameter, are determined. Homogeneous ice nucleation from aqueous (NH4)2SO4 particles 5–39 wt% in concentration and aqueous levoglucosan particles with initial water activities of 0.85–0.99 yield upper limits of the homogeneous ice nucleation rate coefficients of up to 1 × 1010 cm−3 s−1. The experimentally derived homogeneous ice freezing temperatures and upper limits of the homogeneous ice nucleation rate coefficients are compared with corresponding predictions of the water-activity-based ice nucleation theory [T. Koop, B. P. Luo, A. Tsias and T. Peter, Nature, 2000, 406, 611]. It is found that the water-activity-based ice nucleation theory can capture the experimentally derived ice freezing temperatures and homogeneous ice nucleation rate coefficients of the aqueous (NH4)2SO4 and aqueous levoglucosan particles. However, the level of agreement between experimentally derived and predicted values, in particular for homogeneous ice nucleation rate coefficients, crucially depends on the extrapolation method to obtain water activities at corresponding freezing temperatures. It is suggested that the combination of experimentally derived ice freezing temperatures and homogeneous ice nucleation rate coefficients can serve as a better validation of the water-activity-based ice nucleation theory than when compared to the observation of homogeneous ice freezing temperatures alone. The atmospheric implications with regard to the application of the water-activity-based ice nucleation theory and derivation of maximum ice particle production rates are briefly discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...