An ab initio and TD-DFT study of solvent effect contributions to the electronic spectrum of Nile Red†
Abstract
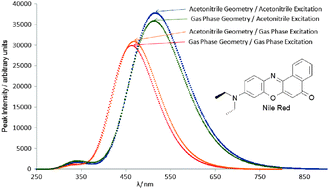
A CIS and TD-DFT study using a polarizable continuum
- This article is part of the themed collection: Time-dependent density-functional theory

 Please wait while we load your content...
Please wait while we load your content...