New insight into the nanostructure of ionic liquids: a small angle X-ray scattering (SAXS) study on liquid tri-alkyl-methyl-ammonium bis(trifluoromethanesulfonyl)amides and their mixtures
Abstract
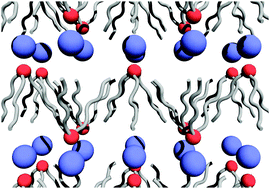
We report a small angle X-ray scattering study on the liquid phase of a series of room temperature

 Please wait while we load your content...
Please wait while we load your content...