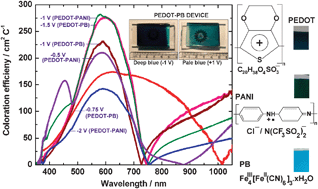
Electrochromic devices based on poly(3,4-ethylenedioxythiophene) (PEDOT) as the cathodic coloring electrode and polyaniline (PANI) or Prussian blue (PB) as the counter electrode containing a highly conductive, self-supporting, distensible and transparent polymer–gel electrolyte film encapsulating an ionic liquid, 1-butyl-1-methylpyrrolidiniumbis(trifluoromethylsulfonyl)imide, have been fabricated. Polarization, charge transfer and diffusion processes control the electrochemistry of the functional electrodes during coloration and bleaching and these phenomena differ when PEDOT and PANI/PB were employed alternately as working electrodes. While the electrochemical impedance response shows good similitude for PEDOT and PANI electrodes, the responses of PEDOT and PB were significantly different in the PEDOT–PB device, especially during reduction of PB, wherein the overall amplitude of the impedance response is enormous. Large values of the coloration efficiency maxima of 281 cm2C−1 (λ = 583 nm) and 274 cm2C−1 (λ = 602 nm), achieved at −1.0 and −1.5 V for the PEDOT–PANI and PEDOT–PB devices have been correlated to the particularly low magnitude of charge transfer resistance and high polarization capacitance operative at the PEDOT–ionic liquid based electrolyte interface at these dc potentials, thus allowing facile ion-transport and consequently resulting in enhanced absorption modulation. Moderately fast switching kinetics and the ability of these devices to sustain about 2500 cycles of clear-to-dark and dark-to-clear without incurring major losses in the optical contrast, along with the ease of construction of these cells in terms of high scalability and reproducibility of the synthetic procedure for fabrication of the electrochromic films and the ionic liquid based gel electrolyte film, are indicators of the promise these devices hold for practical applications like electrochromic windows and displays.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...