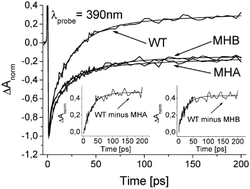
We have recorded transient absorption kinetics at 390 nm with picosecond resolution in order to observe electron transfer from the reduced primary acceptor, A−0, to the secondary acceptor, A1, in wild type and mutated Photosystem I from Chlamydomonas reinhardtii. In the mutants, the methionine axial ligand to the primary electron acceptor in either the A- or B-branch of electron transfer cofactors, was replaced with histidine. Both of the mutations reduced the formation of a positive signal at 390 nm, characteristic of A−1 to a level approximately half of that observed in wild type Photosystem I. It is concluded that in the mutated branch of Photosystem I, electron transfer from A−0 to A1 does not occur. The absorption kinetics resulting from subtraction of either of the mutants’ traces from that of wild type is interpreted to reflect the kinetics of A- or B-side electron transfer from A−0 to A1 in the the wild type Photosystem I. Each of these traces could be fitted with a monoexpoenential decay characterized by the same amplitude and 25–30-ps lifetime. The almost identical effect of both mutations on A−1 formation confirm a similar engagement of both the A- ad B-branches in electron transfer to A1 in Photosystem I from C. reinhardtii. This observation is in contrast to the unidirectional electron transfer concluded from the studies on similar mutants of cyanobacterial Photosystem I.1 Thus, this contribution provides further evidence for functional differences between these two model Photosystems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...