The structural modifications of (S)-1,2,3,4-tetrahydro-3-isoquinoline methanol (THIQM) upon ionisation have been investigated in jet-cooled conditions, by means of laser-induced fluorescence, REMPI, and IR-UV ion-dip spectroscopy of the neutral ground state and the ion. These results combined with ab initio calculations, support the presence in the jet of two low-energy conformers of THIQM. In the most stable Conformer I, the CH2OH substituent acts as a hydrogen bond donor to the nitrogen lone pair in the equatorial position. In this case, the nitrogen atom is in (S) configuration. Conformer II shows the opposite NH⋯O hydrogen bond from the hydrogen atom in the equatorial position of nitrogen to the OH group. In this case, the nitrogen atom is in (R) configuration. This chirality dependence of the hydrogen bond direction is lost upon ionisation. While ionisation of Conformer II reinforces the NH⋯O hydrogen bond, ionisation of Conformer I induces its isomerisation to the same ion as Conformer II, i.e. a change in hydrogen bond direction.
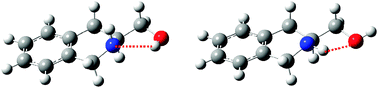
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
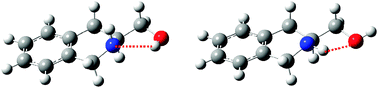

 Please wait while we load your content...
Please wait while we load your content...