The formation of colloidal coppernanoparticles stabilized by zinc stearate: one-pot single-step synthesis and characterization of the core–shell particles†
Abstract
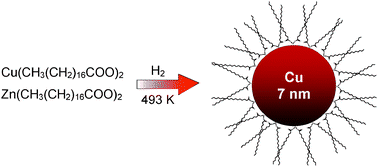
A highly efficient one-step process to generate Cu–Zn colloids was developed, in which the colloidal particles were synthesized from

 Please wait while we load your content...
Please wait while we load your content...