A theoretical investigation of α-Fe2O3–Cr2O3 solid solutions
Abstract
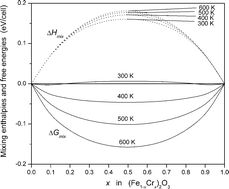
We have examined the thermodynamic stability of α-Fe2O3–Cr2O3 solid solutions as a function of temperature and composition, using a combination of statistical mechanics with atomistic simulation techniques based on classical interatomic potentials, and the addition of a model magnetic interaction Hamiltonian. Our calculations show that the segregation of the

 Please wait while we load your content...
Please wait while we load your content...