Photoinduced intramolecular electron transfer in a 2,7-diaminofluorene chromophore decorated with two benzophenone subunits
Abstract
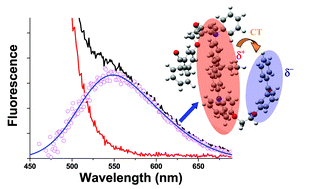
An extensive photophysical analysis of a 2,7-bis-(N-4-methoxyphenyl-N-phenylamino)fluorene derivative covalently linked with two benzophenone moieties is presented. A systematic comparison with a model

 Please wait while we load your content...
Please wait while we load your content...