Wormholes in chemical space connecting torus knot and torus link π-electron density topologies
Abstract
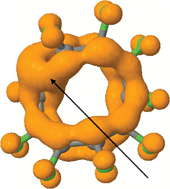
Möbius aromaticities can be considered as deriving from cyclic delocalized π-electron densities ρ(r)π which have the topological form of either a two-component torus link or a single-component torus knot. These two topological forms are distinguished by their (non-zero) linking number Lk, which describes how many times the two components of a torus link cross each other or the single component of a torus knot crosses with itself. The special case of Hückel or benzenoid aromaticity is associated with a π-electron density that takes the form of a two-component torus link for which the linking number is zero. A class of molecule has been identified which here is termed a Janus aromatic, and which bears the characteristics of both a two-component torus link and a single-component torus knot in the topology of the π-electron density. This is achieved by the formation of one (or more) wormholes or throats in the π-electron density connecting the two torus forms, which can impart a Janus-like dual personality to the aromaticity of the system. The impact of such wormholes on the overall π-delocalized aromaticity of such molecules is approximately estimated using a NICS(rcp) index, and subdivides into two types; those where the forms of aromaticity associated with a torus link and a torus knot cooperate and those where they oppose.

 Please wait while we load your content...
Please wait while we load your content...