Co-crystallization experiments conducted between thiocarbamide derivatives, (E)-O-alkyl N-aryl-thiocarbamide, ROC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)N(H)R′, for R = Me, Et and iPr, and R′ = Ph and PhNO2-4, containing a pharmaceutically active chromophorei.e. –C(
S)N(H)R′, for R = Me, Et and iPr, and R′ = Ph and PhNO2-4, containing a pharmaceutically active chromophorei.e. –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–N(H)–, and the bipyridine-type molecules, 4,4′-bipyridine, trans-1,2-bis(4-pyridyl)ethene and 1,2-bis(4-pyridyl)ethane, showed the formation of stable 2 : 1 co-crystals, i.e. {[ROC(
S)–N(H)–, and the bipyridine-type molecules, 4,4′-bipyridine, trans-1,2-bis(4-pyridyl)ethene and 1,2-bis(4-pyridyl)ethane, showed the formation of stable 2 : 1 co-crystals, i.e. {[ROC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)N(H)R′]2(bipyridine-type molecule)}. Novel species were formed by grinding and solvent drop grinding methods, and characterized by powder X-ray diffraction. Five 2 : 1 co-crystals were isolated as single crystals by slow evaporation methods and fully characterized by spectroscopic and X-ray crystallographic methods. Uniformly, trimeric aggregates were formed where each pyridine-nitrogen was connected to an amide-H via a Npyridine⋯H–Namide hydrogen bond. This study shows that the eight-membered {⋯H–N–C
S)N(H)R′]2(bipyridine-type molecule)}. Novel species were formed by grinding and solvent drop grinding methods, and characterized by powder X-ray diffraction. Five 2 : 1 co-crystals were isolated as single crystals by slow evaporation methods and fully characterized by spectroscopic and X-ray crystallographic methods. Uniformly, trimeric aggregates were formed where each pyridine-nitrogen was connected to an amide-H via a Npyridine⋯H–Namide hydrogen bond. This study shows that the eight-membered {⋯H–N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S}2 homosynthon found in the parent thiocarbamides is readily disrupted in the presence of bipyridine-type molecules to enable the formation of the stable heterosynthon, {N⋯H–N}.
S}2 homosynthon found in the parent thiocarbamides is readily disrupted in the presence of bipyridine-type molecules to enable the formation of the stable heterosynthon, {N⋯H–N}.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)N(H)R′, for R = Me, Et and iPr, and R′ = Ph and PhNO2-4, containing a pharmaceutically active chromophorei.e. –C(
S)N(H)R′, for R = Me, Et and iPr, and R′ = Ph and PhNO2-4, containing a pharmaceutically active chromophorei.e. –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–N(H)–, and the
S)–N(H)–, and the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)N(H)R′]2(bipyridine-type molecule)}. Novel species were formed by
S)N(H)R′]2(bipyridine-type molecule)}. Novel species were formed by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S}2 homosynthon found in the parent thiocarbamides is readily disrupted in the presence of
S}2 homosynthon found in the parent thiocarbamides is readily disrupted in the presence of 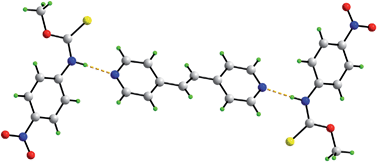

 Please wait while we load your content...
Please wait while we load your content...