Exploitation of an unprecedented silica-promoted acetylene–allene rearrangement for the preparation of C,C-diacetylenic phosphaalkenes†
Abstract
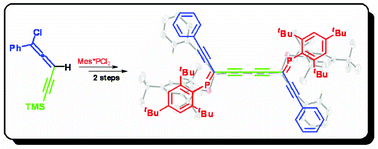
C,C-Diacetylenic phosphaalkenes have been obtained from a 1-chloro-3-ethynyl-1,2-allene which becomes accessible from a silica-promoted rearrangement of a 3-chloropenta-1,4-diyne; oxidative

 Please wait while we load your content...
Please wait while we load your content...