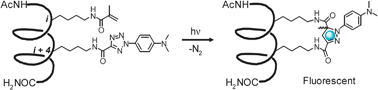
Facile synthesis of stapled, structurally reinforced peptide helices via a photoinduced intramolecular 1,3-dipolar cycloaddition reaction†
Abstract
We report the first use of a photoinduced 1,3-dipolar cycloaddition reaction in “stapling” peptide sidechains to reinforce a model

 Please wait while we load your content...
Please wait while we load your content...