Inversion of product selectivity in an enzyme-inspired metallosupramolecular tweezer catalyzed epoxidation reaction†
Abstract
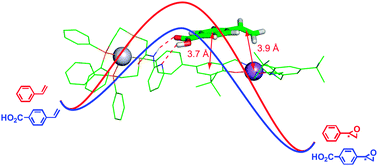
This study describes a heteroligated, hemilabile PtII–P,S tweezer coordination complex that combines a chiral Jacobsen–Katsuki MnIII-salen epoxidation

 Please wait while we load your content...
Please wait while we load your content...