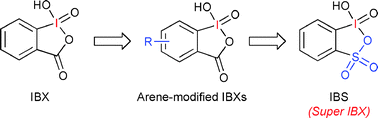
Over the past two decades there has been a dramatic increse in the use of hypervalent iodine compounds in synthetic organic chemistry due to their mild and selective oxidizing properties. Hypervalent iodine compounds catalyze various oxidation reactions such as the oxidation of alcohols, α-oxidation of ketones, oxidative spirocyclization of phenols, etc. Very recently, we found that 2-iodoxybenzensulfonic acid (IBS, 7a), which was generated from 2-iodobenzenesulfonic acidin situ, is an extremely active and mild catalyst for the highly chemoselective oxidation of various alcohols with powdered Oxone® to carbonyl compounds such as aldehydes, carboxylic acids, ketones and cycloalkenones under non-aqueous conditions. In this review, we focus on the design of hypervalent iodine catalysts and review the discovery and development of the oxidation of alcohols with the stoichiometric or catalytic use of hypervalent iodine compounds.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...