Hypervalent iodine reagents as a new entrance to organocatalysts
Abstract
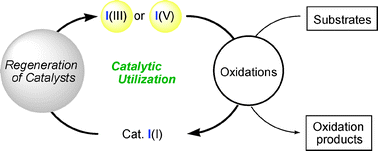
The catalytic utilization of hypervalent iodine reagents, largely in consideration of economical and environmental viewpoints, is a most attractive strategy due to their unique features as extremely useful oxidants, with mild, safe, and environmentally friendly characteristics. In addition to a system based on electrochemical reoxidation conditions, new reliable catalytic methods have emerged in recent years that can broaden the scope of the catalytic concept. For these contributions, a catalytic strategy is now available for performing many representative types of oxidative bond-forming reactions and
- This article is part of the themed collection: ChemComm 60th Anniversary Historic Papers from Japan and South Korea

 Please wait while we load your content...
Please wait while we load your content...