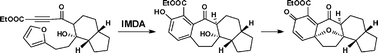
A model study for the concise construction of the oxapentacyclic core of cortistatins through intramolecular Diels–Alder and oxidative dearomatization–cyclization reactions†
Abstract
A unified strategy towards the facile construction of the [6.7.6.5] oxapentacyclic skeleton of cortistatins is reported, featuring intramolecular

 Please wait while we load your content...
Please wait while we load your content...