Phenylenediamine catalysis of “click glycosylations” in water: practical and direct access to unprotected neoglycoconjugates†‡
Abstract
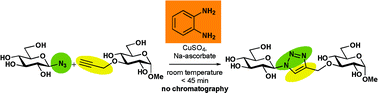
Phenylenediamine-catalyzed click chemistry leads to the efficient, practical, and column-free preparation of neoglycoconjugates from unprotected glucosyl azide, in pure water when

 Please wait while we load your content...
Please wait while we load your content...