6-Azauracil or 8-aza-7-deazaadenine nucleosides and oligonucleotides: the effect of 2′-fluoro substituents and nucleobase nitrogens on conformation and base pairing
Abstract
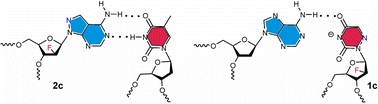
The stereoselective syntheses of 6-azauracil- and 8-aza-7-deazaadenine 2′-deoxy-2′-fluoro-β-D-arabinofuranosides 1c and 2c employing nucleobase anion glycosylation with 3,5-di-O-benzoyl-2-deoxy-2-fluoro-α-D-arabinofuranosyl bromide 6 as the

 Please wait while we load your content...
Please wait while we load your content...