Synthesis of fluorescein-labelled O-mannosylated peptides as components for synthetic vaccines: comparison of two synthetic strategies
Abstract
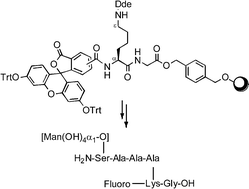
Mannose-binding proteins on the surface of antigen-presenting cells (APCs) are capable of recognizing and internalizing foreign agents in the early stages of

 Please wait while we load your content...
Please wait while we load your content...