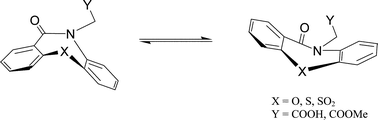
In the perspective of generating libraries for broad screening purposes, the inversion barrier of enantiomeric conformers of various tricyclic compounds, characterized by a seven-membered ring featuring a lactam group with two aromatics condensed at its opposite sides, has been investigated by ab initio calculations and NMR spectroscopy. Solid-state structure characterizations by single-crystal X-ray diffraction have been also carried out. The aim was to assess if moving from ethers to sulfides and sulfones derivatives, causes the racemization barrier to vary. Investigation was also extended to thiolactam congeners. Structural and electronic effects were investigated on the energetics of the inversion process and structure-property relationships evidenced. Results suggest that the ring inversion of the oxo derivatives is easier with respect to the corresponding S/SO2 ones. Also amide molecules have, on the whole, smaller barriers with respect to the corresponding thioamide ones.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...