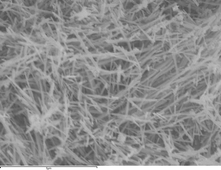
The comparative electrochemical behaviour of both α- and β-nanorods of manganese dioxide (MnO2) and microparticles of predominantly β-phase manganese dioxide is investigated at pHs close to neutral. In order to understand the observed voltammetric behaviour of all three materials the mechanisms of electrodeposition of MnO2 onto a graphite electrode surface from a solution of Mn(II) at pH 3–7 is also reported. It is proposed that two competing mechanistic pathways operate, both invoking MnOOH as an intermediate species, which are an ECE or a DISP process, respectively. At low pH values (<pH 3) the mechanism likely proceeds via a DISP mechanism whereas at high pHs (pH ∼ 7) the mechanism proceeds predominantly as an ECE process with the diffusion of protons out of the MnOOH intermediate being the likely rate determining step.
The α- and β-MnO2nanorods display a ca. 30 mV difference in the observed reduction potentials corresponding to a ca. 3 kJ mol−1 difference in the Gibb’s energy between the two phases. There is also an observable difference in the reduction potential of the β-nanorods and β-microparticles, which probably reflects the differing surface or structural energies of the two materials. Finally the electrocatalytic performance of the three MnO2 materials with respect to the analytical determination of hydrogen peroxide is investigated. Both phases of the MnO2nanorods exhibited a lower limit of detection (5.0 ± 2.5 μM based on 3σ) and greater sensitivity towards H2O2 than the MnO2 microparticles, likely attributed to an increased surface area.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...