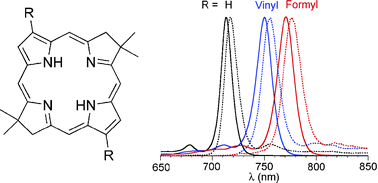
The near-infrared (NIR) spectral region has been comparatively under-utilized for diverse materials and medical applications owing to the lack of chromophores that afford stability, solubility, synthetic malleability, and tunable photophysical features. Bacteriochlorins are attractive candidates in this regard; however, preparation via modification of naturally occurring bacteriochlorophylls or reduction of porphyrins or chlorins has proved cumbersome. To overcome such limitations, a dibromobacteriochlorin (BC-Br3Br13) was prepared de novo by the acid-catalyzed condensation of an 8-bromodihydrodipyrrin-acetal. BC-Br3Br13 bears (1) a geminal dimethyl group in each reduced ring to block adventitious dehydrogenation, and (2) bromo groups at the 3- and 13-positions for further chemical modifications. BC-Br3Br13 was subjected to four types of Pd-mediated coupling reaction (Suzuki, Stille, Sonogashira, dehalogenation) to give bacteriochlorins bearing substituents at the 3- and 13-positions (phenyl, vinyl, acetyl, phenylethynyl), and a benchmark bacteriochlorin lacking such substituents. The 3,13-divinylbacteriochlorin was transformed to the 3,13-diformylbacteriochlorin. Depending on the substituents at the 3- and 13-positions, the position of the long-wavelength absorption maximum (Qy(0,0) band) lies between 713 and 771 nm, the fluorescence emission maximum lies between 717 and 777 nm, and the fluorescence quantum yield ranges from 0.15 to 0.070. The ability to introduce a wide variety of functional groups via Pd-mediated coupling reactions and the tunable absorption and emission spectral properties suggest that synthetic bacteriochlorins are viable candidates for a wide variety of photochemical applications.

 Please wait while we load your content...
Please wait while we load your content...