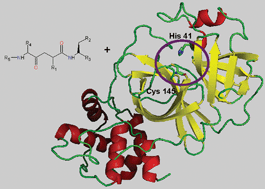
Several outbreaks of Severe Acute Respiratory Syndrome (SARS) have alerted the health systems of the world this century. The treatment of SARS patients with inhibitors, targeting proteins similar to those observed in this coronavirus, has not achieved satisfactory results, and currently there is no effective treatment for its control. The main proteinase of SARS coronavirus (3CLpro or Mpro) is crucial for its replication process, and therefore it is a potential target for the development of anti-SARS drugs. This protease is constituted of two protomers (A and B), displaying an active site formed by the catalytic dyad His-41 Cys-145. In this work, the structure of Mpro (PDB 1UJ1) has been optimized using an MMFF94s force field and docked with AG7088, an inhibitor of the human rhinovirus 3C protease. This protein has structural and functional similarities with SARS Mpro. Docking between AG7088 and the protomers A and B of Mpro, using the FlexX/Cscore program of SYBYL 7.0, showed that the calculated protein–ligand binding affinity was more favorable for the complex with Protomer A than for Protomer B. In order to find a good inhibitor with a high protein binding affinity (BA), 1250 molecules derived from AG7088 were docked with protomer A of Mpro. Molecules (83 inhibitors) with BAs lower than −31 kcal mol−1 (BA of AG7088), and also capable of interacting with the protein within the active site pocket, were considered possible inhibitors of Mpro. The average BA of ligands in all complexes was −36 kcal mol−1, and the best presented a BA of −49 kcal mol−1, suggesting that this example could act as a good Mpro competitive inhibitor. The ligand, named GQAC-02441, was also docked with the non-optimized structure of 1UJ1, generating satisfactory BA values. Taken together, our results propose a new high affinity ligand for SARS Mpro, with inhibitory properties independent of slight structural changes in the protease.

 Please wait while we load your content...
Please wait while we load your content...