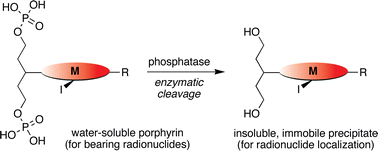
In a new therapy that aims to concentrate and immobilize therapeutic radionuclides in nanoscale assemblies within solid tumors, a soluble precipitable reagent (SPR) is administered as the radionuclide carrier and is converted to non-diffusible precipitate by an enzyme located in tumor tissues. To meet the objective of such an SPR, we have prepared and examined a class of porphyrin–alkyldiphosphates that are soluble in aqueous solution and that are rendered insoluble upon removal of the two phosphate groups. The porphyrins examined herein are of the trans-AB architecture wherein the substituents are a bis(dihydroxyphosphoryloxy)alkyl group and a phenyl (or p-bromophenyl) group. Provisions for later incorporation of radionuclides have been established by preparation of the analogous copper chelate or the meso-iodo free-base porphyrin. Altogether, four porphyrins bearing a bis(dihydroxyphosphoryloxy)alkyl group were examined and found to exhibit satisfactory solubility in water (>1 mM). Dephosphorylation reactions have been carried out in vitro using the enzyme shrimp alkaline phosphatase. In each case, enzyme-induced precipitation was observed. The soluble-to-insoluble conversion has been examined by visual inspection, absorption spectroscopy, electrospray ionization mass spectrometry, and nephelometry using non-radiolabeled porphyrins.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...