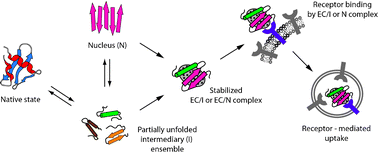
The in vivo formation of fibrillar proteinaceous deposits called amyloid is associated with more than 40 serious human diseases, collectively referred to as protein deposition diseases. In many cases the amyloid deposits are extracellular and are found associated with newly identified abundant extracellular chaperones (ECs). Evidence is presented suggesting an important regulatory role for ECs in amyloid formation and disposal in the body. A model is presented which proposes that, under normal conditions, ECs stabilize extracellular misfolded proteins by binding to them, and then guide them to specific cell receptors for uptake and subsequent degradation. Thus ECs and their receptors may be critical parts of a quality control system to protect the body against dangerously hydrophobic proteins/peptides. However, it also appears possible that in the presence of a high molar excess of misfolded protein, such as might occur during disease, the limited amounts of ECs available may actually exacebate pathology. Further advances in understanding of the mechanisms that control extracellularprotein folding are likely to identify new strategies for effective disease therapies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...