A kinetic study of the phase conversion of layered cobalt hydroxides†
Abstract
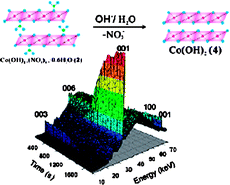
A kinetic study of the phase conversions of three layered α-type cobalt hydroxides, two pink forms {Co(OH)1.4(NCO)0.6·0.6H2O, Co(OH)1.5(NO3)0.5·0.6H2O} and a green form {Co(OH)1.6Cl0.4·0.4H2O} to a brucite-like β-Co(OH)2 in the presence of

 Please wait while we load your content...
Please wait while we load your content...